Study Background:
For detailed information, including potential, societal benefits, etc. please refer to the following documentation: INFORMATION SHEET & INFOGRAPHIC BELOW!
Purpose of the study:
The purpose of this study is to compare the accuracy of CT and intraoperative identification of sentinel (first draining) lymph nodes in dogs with lung tumours and to develop protocols for these methods of staging.
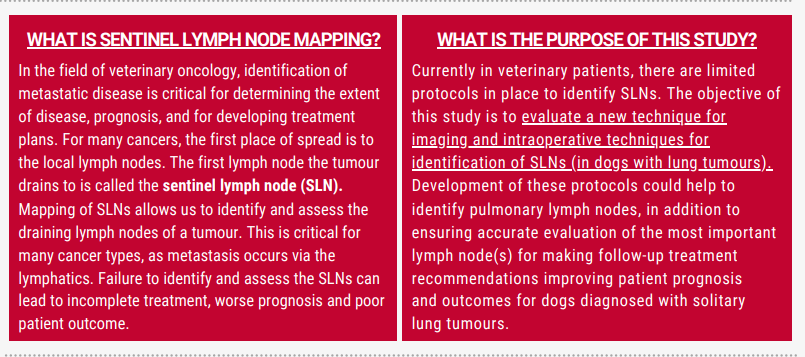
In the field of veterinary oncology, identification of metastatic disease is critical for determining the extent of disease, prognosis, and for developing treatment plans. For many cancer types, metastasis occurs via the lymphatic system and the status of the draining lymph nodes is an important part of the pre- and intraoperative evaluation. The sentinel lymph node (SLN) is the primary lymph node draining the tumour and although one might expect that it is the first lymph node downstream from the tumour, this is not always true. Failure to identify and assess the sentinel lymph node can lead to incomplete treatment, worse prognosis and poor patient outcome.
Currently in veterinary patients, there are not good protocols in place to identify sentinel lymph nodes. The development of these protocols could help us to decrease the number of lymph nodes we remove, in addition to ensuring we are accurately evaluating the most important lymph node
for making follow-up treatment recommendations.
Study Objectives:
The objective of this study is to evaluate a new technique for imaging and intraoperative techniques for identification of sentinel lymph nodes. A secondary objective is to develop a protocol for these techniques in veterinary patients with lung tumours
Study design:
The commitment to participate in this study is no different than if your pet was undergoing a CT scan and surgery for their tumour. In this study, we are considering what is already standard of care (CT and surgery) and trying to improve this process.
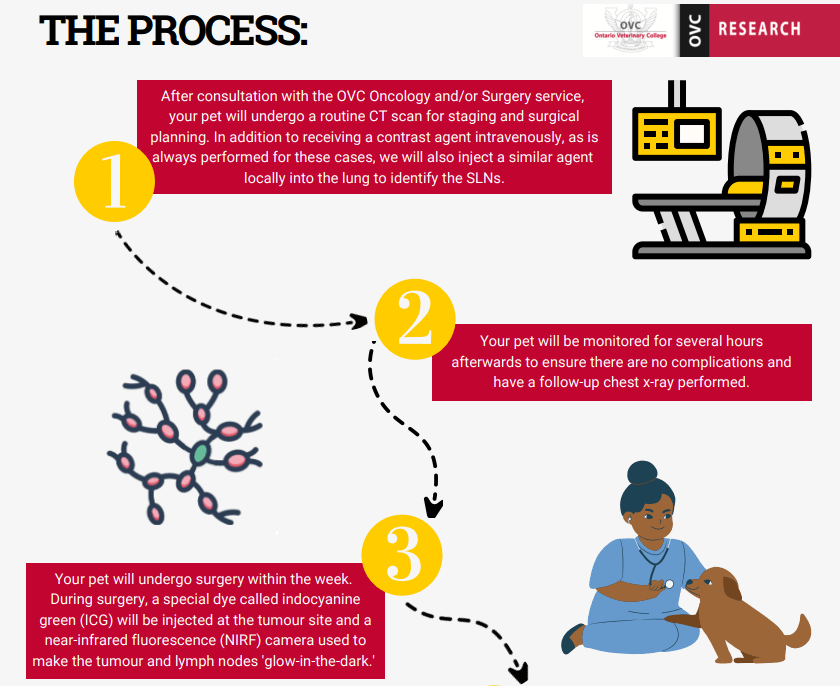
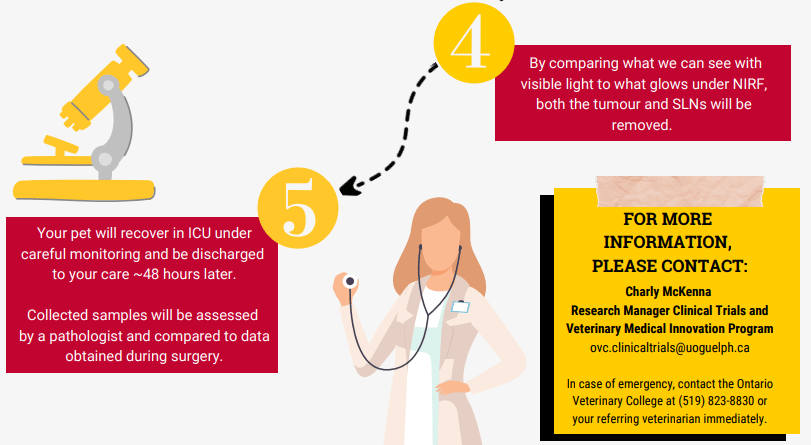
Your pet will present for the CT scan as is routinely arranged by the oncology service. For the CT scan, in addition to receiving a contrast agent intravenously, as is always performed for these cases, we will also inject a similar agent locally. With this injection, we will attempt to identify the lymph node that first drains the tumour, also known as the sentinel lymph node. This information will be compared to our findings in surgery and on histopathology when the tissues are submitted. Following the procedure, your pet will be monitored for several hours to ensure they do not develop any complications from the injection. Surgery will be planned for within 1 week of the CT scan and may be as soon as the day following to ensure that no major changes in the tumour growth have occurred.
Inclusion criteria & eligibility:
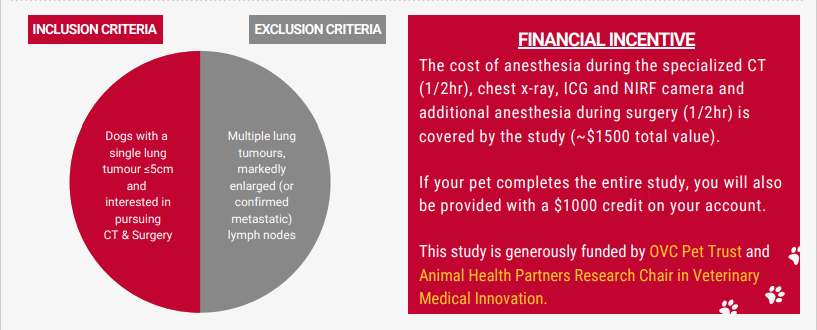
- confirmed diagnosis of a lung tumour undergoing staging and surgery
- patients must be undergoing a CT scan for surgical planning of tumour and lymph node removal.
- The tumour must be removed within 1 week of the CT scan
Incentives:
- Your pet will receive a more detailed CT scan to help with evaluation of the tumour at no additional cost
- The cost of anesthesia during the specialized CT portion (~1/2hr), a chest xray after the CT, as well as the cost of the contrast agents, and the specialized camera to view the contrast during surgery is covered by the study (~$1500 total cost).
- If your pet completes the study you will be provided with a $1000 credit on your account.
Researchers:
- Dr. Michelle Oblak (PI) – moblak@uoguelph.ca
Contact:
Vicky Sabine (PhD), Clinical Research Coordinator
- Work Cell #: 226-218-0338
- Email: ovc.clinicaltrials@uoguelph.ca